by Jim Albert, Eldersburg, MD
The FSHD Clinical Trial Research Network (CTRN) is currently recruiting up to 160 FSHD patients across the seven CTRN sites to participate in a study called ReSOLVE to help standardize a set of tools and measurements for future FSHD clinical drug trials. Recent genetic advances in the understanding of FSHD have identified possible approaches for future drug therapies. It is important that we have appropriate tools in place to help us measure things like strength, function and quality of life which may help us understand if a drug is effective.
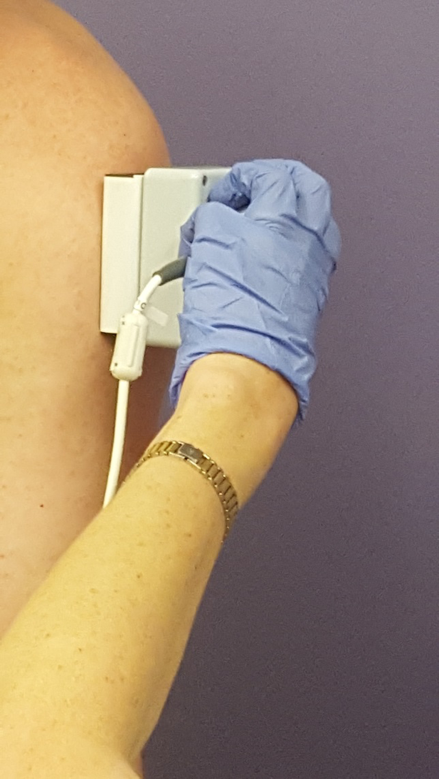
The study aims to validate a new FSHD-specific functional rating scale made up of activities like walking, getting up from a chair, hand strength and a new technique for measuring muscle composition called Electrical Impedance Myography (EIM). EIM is a quick and painless method of measuring muscle composition that can be performed within clinic. A small meter is placed on the target muscle and a measurement is recorded within five seconds using a small electrical current. The study measures eight muscles bilaterally for a total of 16 muscles measured. The targets include three muscles in the arm, three in the leg, one in the back and one in the stomach.
Interaction with a physical therapist is the largest portion of this study. The therapist tests the patient’s ability to walk for six minutes, a short walk starting from both standing and seated , the ability to climb a few stairs, balance along with everyday activities like putting on a coat and picking up a pencil off the floor. Manual muscle testing as scored by the therapist is performed as well as more objective muscle strength testing using a myometer and computer.
A Reachable Workspace Test is performed with the patient sitting in front of a 3-dimensional camera and performing a series of sweeping arm movements in order to test range of motion of the upper extremeties.
The study also measures lean body mass via a DEXA scan (Dual Energy X-ray Absorptiometry). A set of questionnaires are completed that ask questions ranging from scoring the patient’s functional abilities to how FSHD affects the patient’s life. Blood samples are drawn for genetic testing and for storage for future research.
Locations for the study include the seven CTRN locations:
- University of California Los Angeles in Los Angeles, California
- University of Kansas Medical Center in Kansas City, Kansas
- Kennedy Krieger Institute in Baltimore, Maryland
- University of Rochester Medical Center in Rochester, New York
- The Ohio State University in Columbus, Ohio
- University of Utah in Salt Lake City, Utah
- University of Washington in Seattle, Washington
The study involves five visits over a period of 18 months and requires a substantial level of participation by the FSHD patient community in order to reach the goal of 160 participants. According to Kathryn Wagner, MD PhD, director of the Center for Genetic Muscle Disorders at the Kennedy Krieger Institute in Baltimore, MD, “Understanding the natural history of FSHD is a crucial aspect of clinical trial readiness. We are very thankful for the individuals who have stepped up to help us achieve this goal.”
If you live near one of these locations or are willing to travel and you meet the qualifications for the study, please consider participating. Please visit https://clinicaltrials.gov/ct2/show/NCT03458832?cond=fshd&rank=2 for a description of the study and contact information for a site near you.


I live in the Hudson Valley about 4-5 hours from Rochester site. I would be willing to travel.
That’s great, Steven! The study helps to cover at least some of the travel costs.
Here is contact information for the Clinical Trial Readiness study in Rochester, NY.
Contact: Leann Lewis 585-275-7680 leann_lewis@urmc.rochester.edu
Would love to do this sort of thing but I live in the UK ?
I would be willing to participate in this study if the society is interested in picking up my travel cost.I live in Springfield Pa
Please go to clinicaltrials.gov and contact the study site where you would volunteer and ask about coverage of travel costs.
Michael,
It looks like you are near Philly which is only about a 2 hour drive to Baltimore (Kennedy Krieger Instutue… KKI). I’m about an hour from KKI and I get reimbursement for mileage and a meal and I believe there is a separate stipend per visit… something around $50. Those reimbursements come from study funds. I can’t comment on how reimbursement works for those participating that are travelling further form a study site, but you are in a reasonably similar situation as me assuming you would be driving back and forth same day. Although the first visit is a 2 day session with the second day being rather short. After that subsequent visits are 1 day lasting roughly about 6 hours of tests and measurements. So, you might specifically ask how the first visit would go given you might not be too keen on travelling that far two days in a row. For me at an hour drive, it wasn’t a big deal.
Like June said, contact a study site… for you likely KKI… and Geni will contact you to go through a phone-interview to verify that you qualify for the study.
We live on Long Island NY any offsites working with you? St Charles Hospital, Port Jeff?
Unfortunately, at this time there are only the 7 CTRN sites. Some people will travel to participate in a study.
That’s all very interesting. However, I, along with six affected members of my immediate family, all live in wonderful Australia. I shall be interested in your progress. Good luck, and cheers to all involved. Barbara Anderson
I’m willing to participate. Kansas City is a good option.
Hi, my name is Danica Filipovic and I was diagnosed with FSH in 2000 at the age of 22. While I live in Canada, I am willing and able to travel anywhere. Washington State would be my first option, but would consider any other location.
The University of Washington in Seattle is a site for this study!
Hi my name is charle arnold and i live in K.Y and i have fshd for about 13 years
The study includes walking, and climbing stairs. I cannot walk very far, and must use a rollator. I use a powerchair most of the time. Would I be useful for your study?