The FSH Society has awarded a grant for a second year of support for the FSHD Clinical Trials Research Network (CTRN), through the coordinating center based at the University of Kansas Medical Center, Fairway, Kansas. In awarding the grant, the Society notes the value of having a standing, research and trial-ready infrastructure for FSH muscular dystrophy. The CTRN was launched with the help of an FSH Society grant in 2016 (see story), which led to a major NIH UO1 award for important studies to validate clinical trial outcome instruments (see story). Here is a summary of the CTRN:
Facioscapulohumeral Muscular Dystrophy Clinical Trial Research Network (FSHD CTRN). Principal investigator, Jeffrey Statland, MD. Assistant Professor of Neurology, University of Kansas Medical Center, Fairway, Kansas USA.
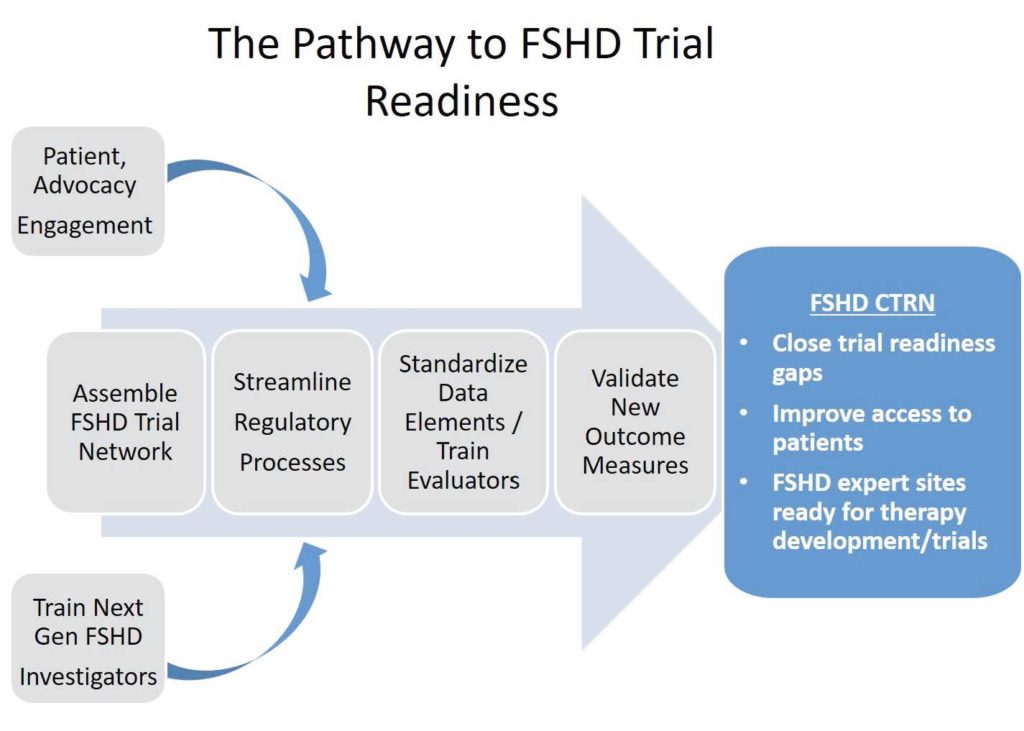
Project Summary. The overall, long term aim of this application is to expedite the development of new therapies for Facioscapulohumeral Muscular Dystrophy (FSHD) by maintaining an FSHD Clinical Trial Research Network (CTRN). Successful clinical trials depend on several factors including: access to and the ability to recruit patients, a precise understanding of the natural history of the disease and the major contributors to disease variability, and reliable outcome measures that are sensitive to change in FSHD. A major hurdle to development of rational trial strategies and validation of outcome measures for FSHD clinical trials is lack of an existing clinical trial network infrastructure with common standard operating procedures (SOPs). We developed the FSHD CTRN to overcome this hurdle by:
- creating a streamlined system for regulatory /ethical oversight;
- developing standards for what data is collected and how it is collected;
- dreating a network of well-trained and knowledgeable clinical evaluators – this is absolutely essential for clinical trials;
- creating a network of trained study coordinators with strong patient engagement, recruitment, and retention skills;
- ensuring the participation of all major stakeholders;
- validating new outcome measures for drug registration studies; and
- training the next generation of FSHD clinical researchers.Not only will the FSHD CTRN help close gaps in trial readiness, but the CTRN also provides a network of sites with a centralized streamlined regulatory process, specific, common expertise in FSHD, and an engaged patient population ready to conduct efficient, high quality clinical trials.


I would like to have infos regarding support groups in south Florida, other patients I can talk to to share our burden
I also would be available for research and trials
I live alone and alone I m facing this upsetting disease
Tks for any help
Maria Rosa Zaniboni
I would like to put you in touch with Ora Prilleltensky and Barbara Dill, two amazing women in Miami who have been organizing our support group meetings there. They have much good advice and are also so supportive of others. May I share your contact information with them?
I also want to encourage you to sign up for our FSHD Family Day conference in Miami, which will be on Saturday, February 23. You’ll have a chance to meet many others who have FSHD, as well as the FSHD care team at the University of Miami Medical School. I will be there as well!
https://www.fshdsociety.org/fsh-events/miami-fshd-family-day-conference/